- My account
-
Cart is empty.
Medical devices
Adaptation to the new Regulation 2017/745 on medical devices
The Regulation (EU) 2017/745 of the European Parliament on medical devices, updates the rules related to the introduction into the market of the European Union (EU), the commercialisation and commissioning of medical devices for human use and their accessories.
In order to achieve maximum quality and safety in its products, Mobercas, S.L. has been adapted to this new regulation to anticipate the deadlines required by law.
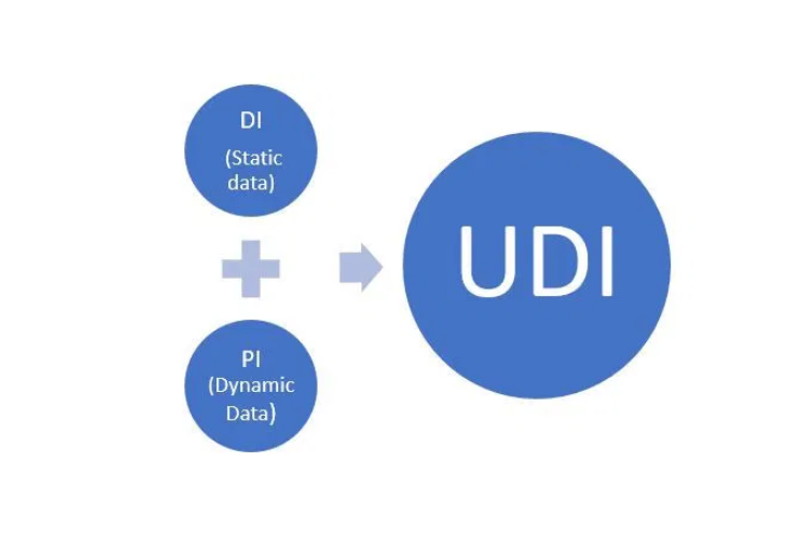
The most considerable changes required by the regulation with respect to the previous Directives are the traceability of Medical Devices through the implementation of a unique identification system or UDI.
UDI encoding
Unique Identification Device or UDI with GS1 standards.
The UDI is a code that unequivocally identifies the medical device throughout the global supply chain. Patients, doctors or any other operator that are part of the chain will be able to identify the UDI information on the label and to access EUDAMED (EMA computer application) to obtain information on the clinical and safety performance of the specific medical device.
The UDI code consists of a fixed device identifier (UDI-DI) and a variable production identifier (UDI–PI). For practical purposes, the UDI-DI is what we have known for years as the “Barcode” or “EAN code” but whose current name is GTIN (UDI-DI).
This information is provided in both human-readable and machine-readable format.

AECOC members
Mobercas S.L. is aware of the importance of product traceability and information transparency so it has partnered with AECOC (an organization accredited by the European Commission as a UDI Issuing Entity) to verify and guarantee the readability of the code by all agents involved in the supply chain.
GS1 CertificateHow do the GS1 standards work?
Effective communication with our customers, partners and suppliers as a crucial and essential part of our company.
Identify
They allow the unique identification of products, logistics units, locations and assets throughout the entire supply chain, from our factory to the consumer.
Capture
GS1 barcodes contain information for traceability and security.
Share
Interoperability, which is made possible thanks to identification, data capture and electronic communication, allows product information to flow throughout the supply chain.